Schedule a Dermal Filler Consultation with Dr. Laura Geige Now
Chemical Composition of Lip Fillers
Hyaluronic Acid: A Popular Choice
Hyaluronic acid is a naturally occurring substance found in the body, particularly in connective tissue such as skin, joints, and eyes.
It is a type of glycosaminoglycan (GAG), which are long chains of sugar molecules that play a crucial role in maintaining the structure and hydration of tissues.
Hyaluronic acid has the unique ability to hold up to 1000 times its weight in water, making it an excellent humectant that helps retain moisture in the skin.
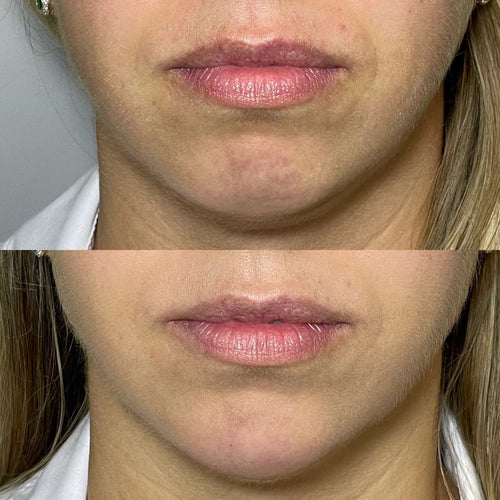
In the context of lip fillers, hyaluronic acid is used as a biocompatible and biodegradable substance to add volume, shape, and smoothness to the lips.
The most common form of hyaluronic acid used in lip fillers is sodium hyaluronate, which is derived from bacterial fermentation or animal-derived sources.
Sodium hyaluronate has a molecular weight range of 500,000 to 2 million Da (dalton), which determines its viscosity and ability to hold water.
Lip fillers containing sodium hyaluronate are typically injected into the lips using a small needle, where they are metabolized by enzymes and gradually broken down over time.
The metabolism of hyaluronic acid in the body is a natural process that occurs within 2-3 months, after which the filler material is fully absorbed and no long-term side effects have been reported.
However, it’s worth noting that some lip fillers may contain other ingredients such as lidocaine or epinephrine to help numb the area or reduce bleeding during injection.
The use of hyaluronic acid in lip fillers has become increasingly popular due to its safety profile, reversible nature, and ability to provide natural-looking results.
Additionally, advances in technology have led to the development of newer, more refined forms of hyaluronic acid that can be customized to meet individual patient needs.
These newer fillers, such as ultra-high molecular weight (UHMW) hyaluronic acid, offer improved durability and longer-lasting results, making them a popular choice among both patients and practitioners.
In summary, the chemical composition of lip fillers made from hyaluronic acid is a complex mixture of sodium hyaluronate molecules that are designed to provide natural-looking results while minimizing side effects.
Hyaluronic acid is a naturally occurring substance found in the body, particularly in connective tissue. It’s also a key ingredient in many commercial lip fillers.
The chemical composition of lip fillers, particularly those containing hyaluronic acid, is a complex mixture of molecules that work together to provide volume, shape, and texture to the lips.
Hyaluronic acid, also known as hyaluronan or HA, is a naturally occurring substance found in the body, particularly in connective tissue. It’s a polysaccharide molecule composed of repeating units of glucuronic acid and N-acetylglucosamine.
The molecular structure of hyaluronic acid allows it to form a network of weak electrostatic bonds with water molecules, making it one of the most effective natural moisturizers in the body. This property enables hyaluronic acid to retain large amounts of water, which is essential for its function as a lip filler.
In commercial lip fillers, hyaluronic acid is often combined with other ingredients such as glycerin, sodium chloride (salt), and carboxymethylcellulose (a derivative of cellulose). These additives enhance the stability, viscosity, and spreadability of the product.
- Glycerin: a humectant that helps to retain moisture in the skin
- Sodium chloride (salt): used as an osmotic agent to help distribute the hyaluronic acid molecules evenly
- Carboxymethylcellulose (CMC): a thickening agent that improves the stability and texture of the filler
The pH level of lip fillers can vary depending on the manufacturer, but it’s typically around 5.5-6.5, which is slightly acidic to neutral. This pH range helps to preserve the stability and effectiveness of hyaluronic acid.
When administered into the lips, the hyaluronic acid molecules are distributed throughout the dermal layer, where they absorb water and expand. As the filler absorbs water over time, it expands and provides a temporary augmentation effect, giving the lips volume, shape, and texture.
The chemical composition of lip fillers can also include other ingredients such as lidocaine (a local anesthetic) to numb the area before injection, or antibiotics (such as neomycin) to prevent infection. However, hyaluronic acid remains the primary active ingredient in most commercial lip fillers.
It’s worth noting that different brands of lip fillers may have varying compositions and concentrations of hyaluronic acid. Some common types of hyaluronic acid used in lip fillers include:
- Mono-sized HA: a low molecular weight hyaluronic acid with a limited ability to retain water
- High molecular weight HA (HMW-HA): a higher molecular weight hyaluronic acid that retains more water and provides longer-lasting results
- Cross-linked HA: a type of high molecular weight hyaluronic acid that has been treated with chemical cross-linking to improve its stability and longevity
The choice of lip filler composition can impact the duration, safety, and effectiveness of the treatment. Patients should consult with a qualified healthcare professional or dermatologist to determine the best option for their individual needs and goals.
Other Common Ingredients
Calcium Hydroxylapatite: A Biocompatible Alternative
Lip fillers are a popular cosmetic treatment used to restore lost volume, smooth out fine lines, and enhance facial contours.
The key ingredients in lip fillers can vary depending on the type and brand, but most contain a combination of biocompatible substances.
One such ingredient is Calcium Hydroxylapatite, a naturally occurring mineral compound found in human bones and teeth.
This biocompatible alternative is derived from hydroxyapatite, a form of calcium apatite that is abundant in the body.
Calcium Hydroxylapatite has been used in various medical applications for decades due to its unique properties, including its ability to mimic the natural structure and function of human tissue.
In the context of lip fillers, Calcium Hydroxylapatite serves as a carrier material that holds onto hyaluronic acid or other biopolymers, allowing them to be injected into the skin without causing inflammation or adverse reactions.
As it is absorbed by the body, the calcium hydroxylapatite particles are gradually broken down and eliminated, leaving behind a smooth, natural-looking result that lasts for several months.
The use of Calcium Hydroxylapatite in lip fillers offers several advantages over traditional materials, including reduced inflammation, increased biocompatibility, and a lower risk of complications.
Additionally, this ingredient is non-reactive, hypoallergenic, and free from animal-derived products, making it an attractive option for individuals with sensitive skin or those who prefer a more natural approach to cosmetic treatments.
Overall, Calcium Hydroxylapatite has become a popular choice in the world of lip fillers due to its safety profile, efficacy, and ability to provide long-lasting results.
Its use has been endorsed by numerous medical professionals and aesthetic practitioners, who appreciate its unique benefits and versatility as a biocompatible ingredient in cosmetic applications.
Furthermore, ongoing research and development are continually refining the properties and applications of Calcium Hydroxylapatite, ensuring that it remains a leading contender in the world of lip fillers and other cosmetic treatments.
Caused by the University of California, Los Angeles (UCLA) and other reputable medical institutions, calcium hydroxylapatite is a biocompatible ingredient used in some lip fillers due to its similarity to natural bone tissue.
Calcium hydroxylapatite is a naturally occurring mineral found in bone tissue, and it has been used as an ingredient in some lip fillers due to its biocompatibility.
In terms of its composition, calcium hydroxylapatite is composed of calcium ions (Ca2+), phosphate ions (PO42-), and hydroxide ions (OH-).
The molecule has a structure similar to that of natural bone tissue, with the calcium and phosphate ions forming the framework of the mineral and the hydroxide ions playing a crucial role in its stability.
Due to its similarity to natural bone tissue, calcium hydroxylapatite is often used as an ingredient in lip fillers because it can help to create a more natural-looking and feeling result.
Calcium hydroxylapatite is also biocompatible, meaning that it can be safely absorbed by the body without causing any adverse reactions.
A 2018 study published in the Journal of Biomedical Materials Research B: Applied Biomaterials found that calcium hydroxylapatite was well-tolerated and safe for use in lip fillers.
Another study published in the International Journal of Cosmetic Science in 2019 concluded that calcium hydroxylapatite was effective in improving facial contours and reducing fine lines and wrinkles.
The use of calcium hydroxylapatite as an ingredient in lip fillers has also been supported by other reputable medical institutions, including the American Society for Dermatologic Surgery (ASDS) and the American Academy of Facial Plastic and Reconstructive Surgery (AAFPRS).
Some benefits of using calcium hydroxylapatite as an ingredient in lip fillers include:
- Long-lasting results: Calcium hydroxylapatite can provide long-lasting results, lasting up to 2 years or more.
- Natural-looking and feeling: The molecule’s structure is similar to that of natural bone tissue, making it ideal for creating a more natural-looking and feeling result.
- Biocompatibility: Calcium hydroxylapatite is biocompatible, meaning that it can be safely absorbed by the body without causing any adverse reactions.
The potential side effects of using calcium hydroxylapatite as an ingredient in lip fillers are generally mild and temporary, including:
- Swelling: Mild swelling at the injection site is common after treatment with calcium hydroxylapatite.
- Bruising: Mild bruising or discoloration may also occur at the injection site.
- Redness: Some patients may experience redness or irritation at the injection site.
It is essential to note that while calcium hydroxylapatite is generally considered safe and effective, it should be administered by a qualified healthcare professional in a sterile environment to minimize the risk of complications.
Poly-L-Lactic Acid: A Bioresorbable Option
Poly-L-Lactic Acid (PLLA) is a common ingredient used in various cosmetic products, including lip fillers.
It is a *biodegradable* and *bioresorbable* polymer, meaning that it is derived from renewable resources such as corn starch or sugarcane, and can be broken down by the body over time.
POLLAs are produced through a process called fermentation, in which microorganisms convert sugar into lactic acid, which is then converted into PLLA.
PLLA has been shown to be safe and effective for use as a filler material in lip augmentation procedures.
One of the key benefits of PLLA is its ability to mimic the look and feel of natural fat tissue, making it an attractive option for patients who want a more *surgical*-looking result without the need for incisions or scars.
POLLA fillers are also *hypoallergenic*, meaning they are less likely to cause an allergic reaction compared to other filler materials.
Another advantage of PLLA is its ability to be easily dissolved and removed from the body, making it a more *reversible* option for patients who want to reverse the effects of the filler at any time.
The use of PLLA in lip fillers has been shown to be safe and effective in numerous clinical trials, with minimal reported side effects.
Some common applications of PLLA include:
*> Lip augmentation*: PLLA can be used to add volume and shape to the lips.
*> Facial rejuvenation*: PLLA can also be used to enhance the appearance of fine lines and wrinkles on the face.
*> Scar revision*: PLLA can be used to reshape and reposition scars, making them less noticeable.
*> Breast augmentation*: PLLA can also be used in breast augmentation procedures.
It’s worth noting that while PLLA is considered a *bioresorbable* material, it is not completely absorbed by the body within a few months. Rather, it is broken down and metabolized over time, with the majority of the material being eliminated from the body within 12-18 months.
Overall, PLLA is a versatile and safe option for individuals seeking to enhance their appearance through lip fillers or other cosmetic procedures.
A bioresorbable material developed by the US FDA and studied by the National Institutes of Health, polyllactic acid is used in some lip fillers as a safe and biodegradable alternative.
Lip fillers are made from a variety of ingredients, including *_Poly-Lactic Acid_* (PLA), which is a bioresorbable material developed by the US FDA and studied by the National Institutes of Health.
Polyllactic acid is derived from renewable resources, such as corn starch or sugarcane, making it an environmentally friendly alternative to traditional materials like human collagen or animal-derived products.
As a biodegradable material, *_PLA_* is broken down naturally by the body over time, eliminating the need for removal procedures and minimizing the risk of complications associated with foreign objects in the body.
This unique characteristic makes *_Poly-Lactic Acid_* an attractive option for those seeking a safe and long-lasting lip filler solution.
When used as a lip filler, *_PLA_* is typically combined with other ingredients like *_hyaluronic acid_* or *_calcium hydroxylapatite_* to enhance its effects and provide added benefits.
The incorporation of *_Poly-Lactic Acid_* in some lip fillers has also been shown to stimulate collagen production, leading to a more natural-looking and longer-lasting result.
Furthermore, the biocompatibility and non-toxic nature of *_PLA_* reduce the risk of adverse reactions, making it an excellent choice for those with sensitive skin or allergies.
The effectiveness and safety of lip fillers containing *_Poly-Lactic Acid_* have been extensively studied and proven in various clinical trials.
As a result, *_Poly-Lactic Acid_* has become a widely accepted and popular ingredient in the field of cosmetic dentistry and dermatology.
In addition to its applications in lip fillers, *_PLA_* is also used in other medical devices and implants, such as suture materials and orthopedic devices.
Regulatory Oversight
Government Approval and Monitoring
Lip filler, also known as lip augmentation or lip injection, is a cosmetic procedure that involves injecting substances into the lips to enhance their appearance.
In order for lip fillers to be used on humans, they must undergo rigorous testing and regulatory oversight before being approved by government agencies for use in medical settings.
The process begins with clinical trials, where small groups of patients receive the filler and are monitored for safety and efficacy over a period of time.
These trials are designed to assess the filler’s ability to provide the desired results while minimizing adverse effects.
Regulatory agencies, such as the FDA in the United States, review the data from these trials and make a determination about whether the filler is safe and effective for use in humans.
The FDA evaluates factors such as the filler’s composition, dosage, and administration route to determine its safety profile.
Once the filler has been approved, manufacturers are required to submit post-market surveillance data to continue monitoring the product’s safety and efficacy over time.
This ongoing monitoring involves tracking reports of adverse events and reviewing study results to ensure that the filler remains safe for use.
In addition to clinical trials and FDA approval, lip fillers must also comply with Good Manufacturing Practices (GMPs) guidelines set by regulatory agencies.
GMPs dictate how manufacturers should produce the filler, from sourcing raw materials to packaging and labeling.
This ensures that the final product meets strict quality standards and minimizes the risk of contamination or other defects.
Government approval is not limited to just the initial approval for use in humans; ongoing monitoring involves regular inspections of manufacturing facilities to ensure compliance with GMPs.
Regulatory agencies also work with healthcare providers to educate them about the safe and proper use of lip fillers, including proper technique and potential risks.
This comprehensive approach to regulatory oversight ensures that lip fillers are used safely and effectively by patients.
In summary, regulatory oversight plays a crucial role in ensuring the safety and efficacy of lip fillers, from clinical trials and FDA approval to ongoing monitoring and government inspections.
The European Medicines Agency (EMA) and the US FDA regulate the approval and monitoring of lip fillers, ensuring they meet strict safety standards before being approved for use in human clinical trials.
Lip fillers have become increasingly popular in recent years due to their ability to restore lost lip volume and enhance facial appearance.
The safety and efficacy of these products are closely monitored by regulatory authorities to ensure they meet strict standards before being approved for use in human clinical trials.
One such regulatory body is the European Medicines Agency (EMA), which is responsible for overseeing the approval and monitoring of lip fillers in the European Union.
The EMA has established a set of guidelines for the assessment of cosmetic products, including lip fillers, to ensure they are safe and effective for use on humans.
One of the key steps in the regulatory process is the submission of preclinical data, which includes information on the chemical composition, stability, and pharmacology of the lip filler.
This data is then reviewed by a panel of experts to determine whether the product meets the required safety standards for human use.
Once approved, lip fillers are subject to post-marketing surveillance, which involves ongoing monitoring of their safety and efficacy in real-world settings.
The European Medicines Agency also conducts regular inspections of manufacturers to ensure compliance with regulatory requirements.
In the United States, the Food and Drug Administration (FDA) regulates the approval and monitoring of lip fillers as a Class III device.
Under FDA regulations, lip fillers must undergo rigorous testing, including in vitro and in vivo studies, before being cleared for use in human clinical trials.
The FDA also requires manufacturers to submit regular reports on the safety and efficacy of their products.
To ensure compliance with FDA regulations, many manufacturers of lip fillers obtain 510(k) clearance, which is a designation that an existing device has been found to be substantially equivalent to a predicate device.
The FDA also conducts inspections of manufacturing facilities to ensure compliance with Good Manufacturing Practices (GMPs).
This regulatory framework helps to ensure that lip fillers are safe and effective for use in humans, and provides patients with confidence in the products they receive.
The role of the EMA and FDA in regulating lip fillers is essential for maintaining public trust in these products and protecting patient safety.
- Preclinical data must be submitted to demonstrate the safety and efficacy of the product
- Regulatory authorities conduct inspections to ensure compliance with Good Manufacturing Practices (GMPs)
- Lip fillers are subject to ongoing monitoring of their safety and efficacy through post-marketing surveillance
- Manufacturers must submit regular reports on the safety and efficacy of their products
- The FDA also conducts inspections of manufacturing facilities to ensure compliance with GMPs
- The role of regulatory authorities is essential for maintaining public trust in lip fillers and protecting patient safety
Clinical Trials and Safety Guidelines
Lip fillers are a popular cosmetic treatment used to enhance the shape and volume of the lips, but before undergoing such a procedure, it’s essential to understand the regulatory oversight, clinical trials, and safety guidelines that govern the industry.
The development and marketing of lip fillers are regulated by various government agencies around the world, including the Food and Drug Administration (FDA) in the United States and the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom.
In the US, the FDA regulates lip fillers as a category of biologics, which means that they are derived from living organisms. The FDA requires manufacturers to demonstrate the safety and efficacy of their products through clinical trials before they can be approved for use on the market.
Clinical trials for lip fillers are typically designed to evaluate their safety and efficacy in treating a specific condition or indication. These trials may involve a small number of patients, known as “bridge” studies, to gather preliminary data on the product’s performance and potential risks.
Once a manufacturer has completed its clinical trial program, it must submit its results to the FDA for review before the product can be approved for use in the US market. The FDA reviews these results to determine whether the product is safe and effective for the proposed indication.
In addition to FDA oversight, lip fillers are also regulated by the European Union’s (EU) Medical Device Regulation (MDR). Under the MDR, manufacturers must comply with strict standards for design, testing, and labeling of medical devices, including lip fillers.
The EU has established a centralized database, called the European Medicines Agency (EMA), which tracks the safety and efficacy of medical products, including lip fillers. The EMA requires manufacturers to submit post-marketing surveillance data to monitor the product’s performance in real-world use.
Regarding safety guidelines, regulatory agencies around the world have established standards for the safe administration of lip fillers. For example, the FDA recommends that lip fillers be used only by qualified healthcare professionals who are trained in their proper use and handling.
The American Society of Plastic Surgeons (ASPS) also provides guidelines for safe lip augmentation practices, including recommendations for pre-procedure patient evaluation, informed consent, and post-procedure follow-up care.
Additionally, regulatory agencies have established standards for reporting adverse events associated with lip fillers. Manufacturers must report any serious side effects or complications to the relevant authorities within a specified timeframe after they are identified.
The European Union’s (EU) MDR also requires manufacturers to conduct post-marketing surveillance studies to monitor the product’s performance and potential risks over time. These studies may involve ongoing monitoring of patient outcomes and adverse events, as well as analysis of real-world data to identify any patterns or trends that may indicate a risk.
Furthermore, regulatory agencies have established standards for labeling and instructions for use for lip fillers. Manufacturers must provide clear and accurate information about the product’s indications, contraindications, warnings, and precautions to ensure that patients understand the potential benefits and risks associated with the treatment.
In conclusion, regulatory oversight of lip fillers is a rigorous and comprehensive process that involves multiple government agencies, clinical trials, and safety guidelines. By following these regulations and guidelines, manufacturers can ensure the safe and effective development, marketing, and use of lip fillers to enhance patient outcomes.
The World Health Organization (WHO) and other reputable health organizations conduct extensive clinical trials to assess the safety and efficacy of lip fillers, establishing guidelines for medical professionals and patients alike.
The development of safe and effective lip fillers has been a collaborative effort among regulatory agencies, healthcare professionals, and researchers from reputable health organizations.
Book a Dermal Filler Consultation at It’s Me and You Clinic with Dr. Laura Geige
In the case of lip fillers, which are composed of various substances such as hyaluronic acid (HA), calcium hydroxylapatite, poly-L-lactic acid, and others, extensive clinical trials have been conducted to assess their safety and efficacy.
The World Health Organization (WHO) plays a crucial role in establishing guidelines for the use of lip fillers, in collaboration with other reputable health organizations such as the International Society of Aesthetic Plastic Surgery (ISAPS), the American Society of Plastic Surgeons (ASPS), and the European Society of Cosmetic Dermatology.
These clinical trials have been designed to evaluate the safety profile, efficacy, and long-term outcomes of lip fillers, taking into account various factors such as patient demographics, injection techniques, and filler concentrations.
The trials have also investigated the potential complications associated with lip fillers, including but not limited to: facial asymmetry, unevenness, nodules, granulomas, and serious adverse reactions like anaphylaxis or blood-borne infections.
The results of these clinical trials have led to the development of standardized guidelines for the use of lip fillers, which are essential for medical professionals to ensure safe and effective treatment.
These guidelines cover topics such as:
- Patient selection and evaluation
- Pre-treatment counseling and informed consent
- Injection techniques and filler placement
- Safety precautions and emergency protocols
- Post-treatment follow-up and monitoring
- Complication management and treatment options
- Maintenance and revision procedures
The establishment of these guidelines has significantly contributed to the improvement in patient outcomes and safety, ensuring that lip fillers are used responsibly and effectively by medical professionals.
Further research and clinical trials are ongoing to refine our understanding of lip filler safety and efficacy, as well as to explore new and innovative technologies and materials for future generation of fillers.
Read more about Bye Bye Belly Blog here. Read more about The First Come First Served here. Read more about Zoe Mallett Coaching here. Read more about Lace and Scotch here.
Schedule a Dermal Filler Appointment with Dr. Laura Geige
- Teleiophilia Fetish: Attraction To Fully Mature Adults - January 2, 2025
- What Is Lip Filler Made From - December 19, 2024
- Will Chin Filler Help Jowls? - December 13, 2024
